Background: PCL is a rare and aggressive form of multiple myeloma (MM). It is associated with high risk cytogenetics, aggressive disease biology and a dismal prognosis. Dara has revolutionized the treatment of MM leading to deep and durable responses as a single agent and in combination with other agents. We evaluated the efficacy of Dara-based regimens in the treatment of PCL.
Methods: Clinical charts of primary and secondary PCL patients treated at the Mayo Clinic Cancer Center between 2012-2019 were reviewed. Survival was analyzed with the Kaplan-Meier method. Plasma cell leukemia was defined by ≥5% peripheral blood plasma cells and/or absolute plasma cell count ≥0.5 x109/L (based on IMWG consensus statement).1
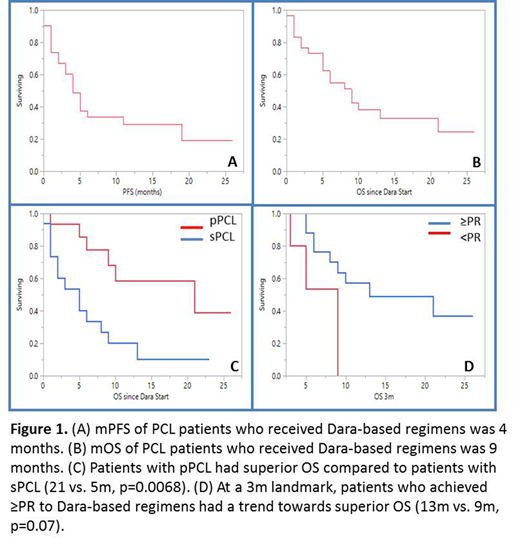
Results: Thirty-one patients were identified (55% female) with a median age at PCL diagnosis of 66 y (range 38-83). Fifteen (48%) had primary (p) PCL, while 14 (52%) had secondary (s) PCL. Nineteen (61%) had high risk cytogenetics with 11 (45%) del 17p, 4 (13%) t(14;16), 14 (45%) 1q amplification/duplication and 3(10%) t(4;14). Twelve (39%) had t(11;14). Eighteen (58%) patients had undergone autologous hematopoietic cell transplant (AHCT) with 12 (39%) having undergone AHCT following their diagnosis of PCL. Patients received a median of 2 prior lines of therapy (range 1-9) prior to receiving Dara-based regimens; 15 (42%) were refractory to an immunomodulatory agent (IMiD), 18 (58%) were refractory to a proteasome inhibitor (PI) and 10 (32%) were refractory to both. Twenty-eight (90%) were IMiD exposed, 31 (100%) were PI exposed. Eighteen (58%) were lenalidomide (R) refractory, 5(16%) were pomalidomide (P) refractory, 8 (26%) were carfilzomib (K) refractory and 13(42%) were bortezomib (V) refractory prior to receiving Dara. Four (13%) received Dara + chemotherapy, 8 (26%) received Dara + PI, 11 (35%) received Dara + IMiD, 5 (16%) received Dara + PI + IMiD and 4 (13%) received single agent Dara. Median follow up time was 26 months (m) (95% CI; 13-61) from diagnosis of PCL and 17m (95% CI; 10-23) from initiation of Dara. Overall response rate (ORR) to Dara-based regimens was 65% (20/31 ≥partial response). The median duration of response was 5.5m (95% CI; 5.6-12.7). The median time to first response was 1m (95% CI; 0.57-1.72) and the median time to best response was 1m (95% CI; 0.7-2.5). Since the start of Dara-based treatment, median progression free survival (PFS) was 4m (95% CI; 2-11, Figure 1A) and median overall survival (OS) was 9 m (95% CI; 5-21, Figure 1B). Patients with pPCL had superior PFS (19 m vs. 3m, p=0.0096) and OS (21 vs. 5m, p=0.0068, Figure 1C) following Dara-based treatment compared to those with sPCL, respectively. Being refractory to an IMiD, PI or both when receiving Dara did not affect PFS or OS. Patients with high risk cytogenetics had similar OS as compared to those with standard risk cytogenetics (8m vs.13m, p=0.89) following Dara-based treatment. After performing a landmark survival analysis at 3m, achieving ≥PR to a Dara-based regimen resulted in a trend towards superior OS (13m vs. 9 m, p=0.07, Figure 1D). Patients who underwent AHCT following the diagnosis of PCL had a superior OS (41m vs. 6m, p=0.020) however there were no PFS or OS differences whether Dara-based regimens were given before or after AHCT.
Conclusions: Dara-based regimens induce a high ORR in patients with PCL with a rapid time to first response. However, the OS of patients with PCL, particularly sPCL remains dismal. Prospective trials for PCL patients using novel therapeutic strategies are warranted.
1Fernandez de Larrea, et al. Plasma Cell Leukemia: consensus statement on diagnostic requirements, response criteria, and treatment recommendations by the International Myeloma Working Group (IMWG). Leukemia. 2013; 27(4): 780-791.
Alhaj Moustafa:Acrotech: Consultancy. Kapoor:Janssen: Research Funding; Sanofi: Consultancy, Research Funding; Cellectar: Consultancy; Celgene: Honoraria; Amgen: Research Funding; Takeda: Honoraria, Research Funding; GlaxoSmithKline: Research Funding. Dingli:Millenium: Consultancy; Sanofi-Genzyme: Consultancy; Rigel: Consultancy; Bristol Myers Squibb: Research Funding; Karyopharm Therapeutics: Research Funding; Janssen: Consultancy; Alexion: Consultancy; Apellis: Consultancy. Ailawadhi:Phosplatin: Research Funding; Medimmune: Research Funding; BMS: Research Funding; Cellectar: Research Funding; Pharmacyclics: Research Funding; Janssen: Research Funding; Takeda: Honoraria; Amgen: Research Funding; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal